
Top stories






More news
















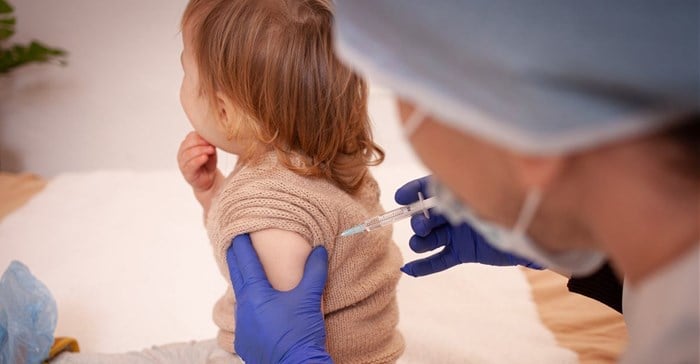
This is even though it's been reported two doses failed to produce the hoped-for immune response among children aged two to four in a clinical trial.
The FDA will probably decide on an approval this month.
This would mean that children under the age of five in the USA could soon be receiving their Covid-19 vaccinations.
After approval, a vaccine dose will contain 10% of the active ingredient that people from the age of 12 get.
Pfizer-BioNTech said they now continue to review the third dose in clinical trials while commencing the FDA process.
According to the US Food and Drug Administration, children as young as six months old can receive Covid-19 vaccinations.
It is considered unusual that the authority had asked the two companies to submit the application for a vaccination with two doses earlier than originally planned by Pfizer and Biontech.
It would be the first US-approved vaccine against Covid-19 for children under the age of five.
Trials are also currently underway for a three-dose vaccine.
Just more than 20 million children in the United States are under the age of five.