#AfricaMonth: Injecting the continent - and the world - with hope

When will the first South African mRNA vaccine be ready for release to the market?
Petro Terblanche: We're probably two years away.
Is Afrigen South African?
Terblanche: Afrigen is truly, proudly South African. We're actually a Level 1 BEE company; we are half-owned by the IDC - the Industrial Development Corporation (35%) - and 65% by Avacare Healthcare Group, the SA-based pan-African company. What we've built here at Afrigen is a manufacturing facility for vaccines end to end - from the sequence right through to the final vaccine that can go into people's arms.
In terms of Afrigen, are there any SA biotech companies competing with you?
Terblanche: No. We are the first and the only mRNA company on the continent.
How did Covid-19 galvanise you to reach this turning point in your career? How did the early days of the lockdown challenge you to find a solution to the pandemic?
Terblanche: Covid was an incredible motivation and inspiration to do something. We're a young company, pre-revenue earning, and in the start-up phase. We have a portfolio of vaccines we're working on that's going to take a long time to get to markets, but when the pandemic started, we decided to diversify our portfolio, and use our existing knowledge base because mRNA is the future-relevant platform to be in.
Three weeks before the World Health Organisation (WHO) put out the call, we had literally just started building a facility for mRNA. We had the strategic foresight to decide to start building.
At the time, we didn't have a product, we didn't have a process, but we told ourselves: "We will learn how to make this mRNA vaccine; we're building a facility, because without a facility we cannot do anything." It was risk taking, but it was also visionary.
Most people will tell you that is absolutely unprecedented, that you have to have a business plan, the business has to bank, you have to have off-take agreements, and a route to market. We didn't have that. All we had was a shareholder who said, "Strategically you're right; go for it."
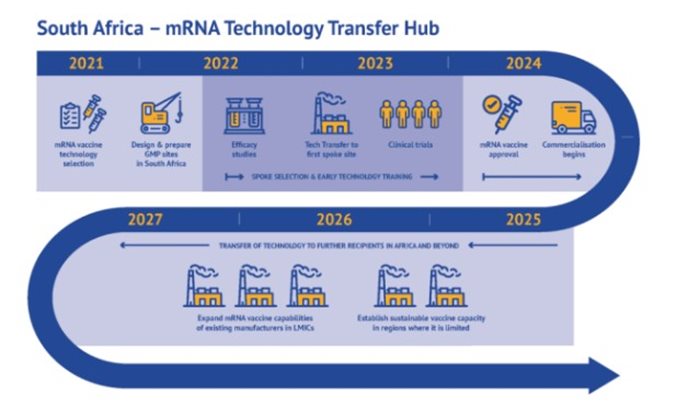
And since the announcement on 21 June 2021 by WHO that the consortium Afrigen Medical Research and Biovac will be the technology transfer host - Afrigen the hub, Biovac the first spoke, with the Medical Research Council managing the clinical trials and the R&D programme - our lives changed.
What is the cost of the project?
Terblanche: The total cost of the project over five years is €103m. This project is fully funded by governments and philanthropic organisations. It's grant funding. It's structured and funded by the French government, the Belgian, Canadian, South African and Norwegian governments, the European Commission, and now we're waiting to hear from a few philanthropic organisations on whether they will support.
What was the role of Wits University in this development?
Terblanche: This is a consortium - a team effort between Wits University and ourselves. Wits is critical in this. Wits worked on RNA for many years for therapeutic uses, and when we couldn't get the tech transfer, we went to Wits. Wits, however, couldn't formulate and we had formulation capability, so the two groups together created the new vaccine. This is a public-private university partnership. It's beautiful.
It demonstrates the science-base in South Africa. The science base in Africa is very strong and the comments made by the world that we can't transfer technology to Africa because they don't understand science is nonsense. The team here understand science very well.
Being a woman in this field is a huge achievement. What do you feel you've brought to the industry that maybe a man couldn't have?
Terblanche: Nothing. Because we generalise so easily. It's interesting. If you look at Afrigen, we're quite female [dominated]. We're 60% women and in fact the three bio-processing engineers on our staff are all women. We have a few chemical engineers, and so on... but we are female dominated. In a way, the [Science Health sector] is becoming more female dominated.
I think what we bring to the sector is passion. We tend to be emotional - we bring passion. This is not only hard business for me. Yes, it has to be sustainable but this is business for me that changes a landscape, so this project has become a passionate project to empower our own people to make their own vaccines, and for us not to sit and wait for the world to have enough to give to us.
It must be hugely empowering...
Terblanche: Yes. What this project draws from me is all my experience in all these years in technology: IP, strategy, business, finance, product development, politics, influence, public relations. This project just pulls it all out, and I think that's why I'm enjoying it so much.
What changes have since come about since WHO's announcement?
Terblanche: The team has been through an incredibly steep learning curve. We went from 12 people to 55. We were in one warehouse; now we're in four warehouses.
We have tripled our capacity, and at the same time we have started transferring knowledge to other spokes. The 16 other organisations that are part of this network in LMICs [low and middle income countries] will also have end-to-end full vaccine-manufacturing capacity.
Everything we develop and learn, all the analytical requirements and the product specifications, we share with over 16 companies in LMICs to make sure they can make mRNA vaccines: the first one Covid-19, and the second one will be strategically determined.This is a paradigm shift [particularly in contrast] to the big-industry sector which doesn't want to share knowledge. BioNTech, Moderna, even J&J - they don't want to share [their intellectual property], but here's this little biotech company that has to be sustainable - and commercially competitive - and we go full out to share [our knowledge].
Where will the first factories start up?
Terblanche: So Biovac are making other vaccines - but they will be the first to make mRNA vaccine. Kenya, Nigeria, Senegal, Egypt and Tunisia are the five countries that will receive mRNA transfers from this hub.
How many factories will there be - one per country?
Terblanche: We have to be careful of the balance, that we don't over-capacitate, that we don't oversupply because then you have wastage; it has to be very carefully balanced in terms of the capacity we're creating.
Given that there will be these sheer quantities produced, means that there will be a demand, so you don't see Covid going away?
Terblanche: I see Covid going away; in fact we hope it will be gone soon, but I also see us requiring annual Covid boosters like annual influenza boosters. I believe we're in a cycle for boosters, and already the big pharmaceutical companies are already changing their vaccines to be booster vaccines because that is where the market is.
This programme is building platforms that will not only make [mRNA] Covid vaccines - Covid is our demonstration project. We will have mRNA vaccines for HIV, TB, malaria, vaso [sickle cell disease], dengue fever, Ebola - because mRNA lends itself towards those kinds of vaccines.
 What does this mean for Africa and vaccine equity? I'm assuming that Afrigen will be serving Africa first and then the rest of the world. Will there be capacity for such global distribution one day?
What does this mean for Africa and vaccine equity? I'm assuming that Afrigen will be serving Africa first and then the rest of the world. Will there be capacity for such global distribution one day?Terblanche: Yes, so you can imagine this is a complex project. It requires an ecosystem of procurement culture and logistics and regulatory [policy] to be in place. So the first spoke in Africa that will get off the ground will be Biovac (they're here in Cape Town).
There are [spokes in] five African countries but we're running parallel with the Eastern and South American companies; so the 15 companies - depending on the readiness of the company - will get on-stream for production almost parallel depending on capacity.
If Afrigen weren't here, what would the implications have been otherwise for Africa?
Terblanche: If Afrigen wasn't appointed as the hub, there would be another company [doing so] because it was a competitive process. There were 56 applications. However, whether the company would have the same business approach I don't know, because our process is quite strategic.
Another component of this programme is that we need to keep these 16 companies running and producing vaccines so that when a [new] pandemic starts we can switch [production] and within 100 days make a vaccine that's relevant for the pandemic. So this part of the programme falls part of WHO's pandemic preparedness goal.
If there hadn't been this capacity, Africa would just have continued to import from Pfizer and Moderna, and continued to buy from them when they have vaccines.
This programme fundamentally wants to ensure we can make our own vaccines. In Africa, about 1% of the vaccines we use are produced here. This kind of project will take it to 40 or 60%.
If Afrigen weren't here, we would never have gotten the opportunity to make mRNA vaccines, maybe other vaccines, but not mRNA.
We have this opportunity to help the world, and to do it on a big scale.
















