
Top stories





Energy & MiningGlencore's Astron Energy gears up with new tanker amidst Sars dispute
Wendell Roelf 9 hours

More news

















Logistics & Transport
Uganda plans new rail link to Tanzania for mineral export boost











Scientists at Southern University of Science and Technology in Guangdong, China, condemned He’s research asserting he “has seriously violated academic ethics and codes of conduct,” and philosophers and bioethicists were quick dive into the morass of editing human genomes. So I’m not going to cover that territory. What I want to address is what we learned: how He made these babies.
I am theoretically a retired professor in the Department of Biomedical Sciences at Colorado State University. For more than 50 years, I have researched numerous aspects of assisted reproductive technology including cloning and making genetic changes to mammalian embryos, so I am interested in most any research concerning “designer babies” and the health problems they may suffer.
At the conference He gave a general overview of the science. While research like this would typically be presented to the scientific community by publishing in a peer-reviewed journal, which He claims that he intends to do, we can get a rough sense of how he created these modified babies. This is something that has been successfully done in other species and just last year in human embryos – but the latter were not implanted into a woman. He says he spent three years testing the procedure on mice and monkeys before he moved to working on human embryos.
There is no doubt that precise genetic modifications can be made to human sperm, eggs, embryos and even some cells in adults. Such modifications have been done ad nauseum in mice, pigs and several other mammals. Thus, it is obvious to scientists like myself that these same genetic modifications can, and will, be made in humans. The easiest way to make genetic changes begins with the embryo.
The trendiest strategy to modify DNA these days involves the CRISPR/Cas-9 gene editing tool, which can make precise genetic modifications in living cells. Although other tools have been available for years, the CRISPR/Cas-9 approach is simpler, easier, more accurate and less expensive.
The way it works is simple in concept. The Cas-9 component is a molecular scissors that cuts the DNA at the location specified by a small piece of RNA, called the “CRISPR template.” Once the DNA is cut, a gene can be modified at that location. The cut is then repaired by enzymes already present in cells.
In this case, He targeted a gene which produces a protein on the surface of cells called CCR5. The HIV virus uses this protein to attach to and infect the cell. He’s idea was to genetically change CCR5 so that HIV can no longer infect cells, making the girls resistant to the virus.
At this point He has not provided a clear explanation of exactly how he disabled the CCR5 and the nature of the genetic modification. But this kind of “disabling” is routinely used in research.
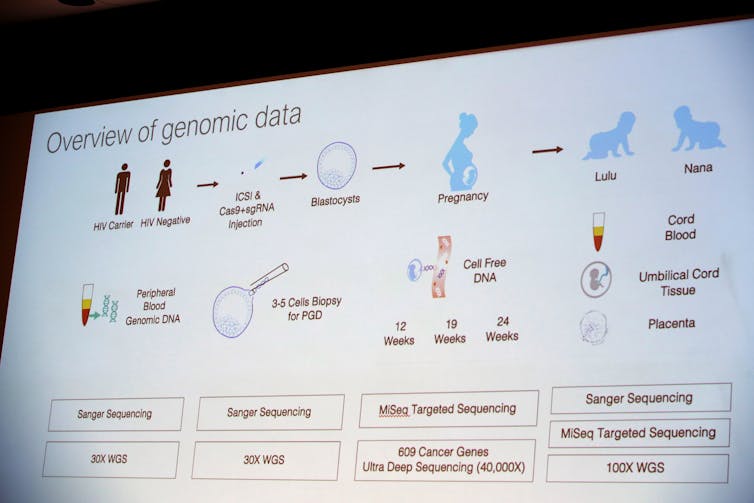
From the diagram He presented, it appears that He injected the CRISPR/Cas-9 system into an egg at the same time as he injected a sperm to fertilise it. After this, the egg divided and formed a ball of dozens of cells – the embryo. At this stage, He removed a few cells from each embryo to determine if the desired genetic change was made. Based on my experience, the embryos were probably frozen at this point. When the analysis was complete, He probably thawed the modified embryos and transferred the best ones back into the mother’s uterus for gestation to term. Embryos without the edits or incorrect edits would either be discarded or used for research.
For many applications, it is ideal to make any changes to the genes at the one-cell stage. Then, when the embryo duplicates its DNA and divides to make a two-cell embryo, the genetic modification is also duplicated. This continues so that every cell in the resulting baby has the genetic change.
However, it appears that the genetic modification in this case did not occur until the two-cell stage or later, because some cells in the babies had the modification, while others did not. This situation is called mosaicism because the child is a mosaic of normal and edited cells.
What could go wrong in a gene-edited embryo? Plenty.
The first glitch is that no modification was made, which occurs frequently. A variation is that the change occurs in some cells of the embryo, but not in all the cells, as occurred in these babies.
The most common worry is so-called non-target effects, in which the genetic modification is made, but other unintended edit(s) occur in other locations in the genome. Having a modification at the wrong place can cause all kinds of developmental problems, such as abnormal organ development, miscarriage and even cancers.
From his slide it appears that He sequenced the genomes – the complete genetic blueprint for each child – at multiple stages of the pregnancy to determine whether there were any undesirable modifications, though these aren’t always easy to find. But until independent scientists can examine the DNA of these two baby girls, we won’t know the results. It is also not clear from the results He has shared so far whether this genetic change can be transmitted to the next generation.
Another common problem already alluded to is mosaicism, which appears to have happened in one of these twins. If some cells are edited, and some not, the baby might have liver cells that contain the edited gene and heart cells that have the normal version, for instance. This may or may not lead to serious issues.

Another issue is that manipulating embryos in vitro – outside their normal environment in the reproductive tract – where we can’t precisely duplicate the normal nutrition, oxygen levels, hormones and growth factors – could lead to developmental abnormalities including oversize fetuses, metabolic problems, and so on. This sometimes occurs with routine procedures such as in vitro fertilization when there is no attempt to make genetic modifications.
Fortunately, nature is quite good at weeding out abnormal embryos via embryonic death and spontaneous abortion. Even in healthy human populations reproducing normally, nearly half of embryos die before the woman even knows that she was pregnant.
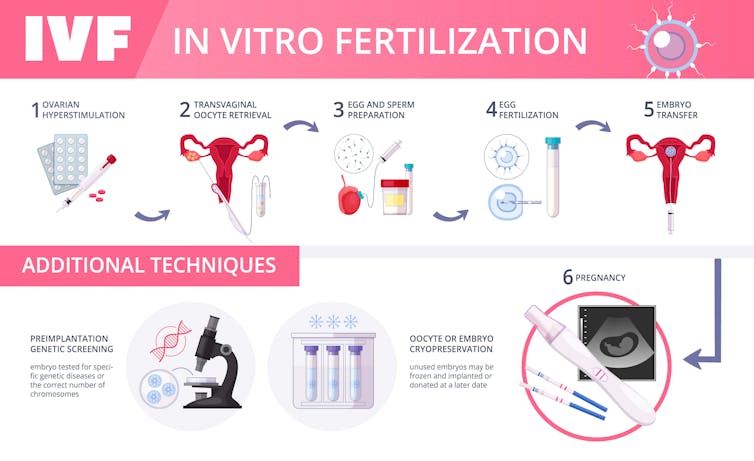
While I have emphasised what can go wrong, I believe that the science will evolve such that genetically modified babies will be healthier than unmodified ones. And these improvements will be passed on to future generations. Severely debilitating genetic abnormalities such as Tay-Sachs syndrome could be removed from a family by genetic modification.
Arguably, designer babies are already being born using a technique called pre-implantation genetic diagnoses (PGD). A few cells from embryos are screened for dozens, and potentially hundreds, of genetic abnormalities such as Down syndrome, cystic fibrosis and Tay-Sachs syndrome, to name a few. Parents are also able to choose those embryos of the desired sex. In my view, choosing which embryos to implant is clearly making designer babies.
Going a step further, PGD isn’t restricted to just eliminating disease. A prospective parent can also choose other traits. When one of the prospective parents in infertile, there are catalogs that provide the race, height and weight, and even the educational level of a sperm or egg donor, who is also determined to be free of major genetic defects, and free of AIDS and other venereal diseases.

In my opinion, if the procedures are deemed ethically and morally acceptable, most genetic modifications likely to be made editing embryos as He says he has done, will involve removal of harmful traits rather than adding desirable ones. Because the changes will be targeted, they will be more precise and less harmful than the mutations that occur randomly in DNA of essentially all sperm and eggs naturally.
With all of this reproductive technology, there is one other consideration: the huge costs of the procedures described. To what extent should society invest scarce medical resources in applying such techniques, especially since any benefits likely will accrue mostly to wealthier families?
These perspectives need to be kept in mind when evaluating potential genetic manipulations of humans.
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()

The Conversation Africa is an independent source of news and views from the academic and research community. Its aim is to promote better understanding of current affairs and complex issues, and allow for a better quality of public discourse and conversation.
Go to: https://theconversation.com/africa