
Top stories






More news

















Logistics & Transport
Uganda plans new rail link to Tanzania for mineral export boost








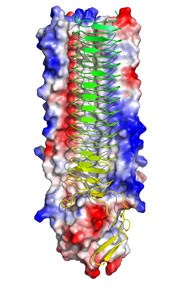
The research, led by a team of biochemists, microbiologists and physicists and published in the Proceedings of the National Academy of Sciences (PNAS), provides an unprecedented level of detail of the consequences of a bacterium approaching another cell, directly in situ.
Until now, traditional approaches to understanding infection have focused on either studies of the cells involved or dissection of individual molecules present within the cells. Leo Brady, Professor of Biochemistry and Mumtaz Virji, Professor of Molecular Microbiology, who led the research, have developed a novel method for bridging these, until now, separate approaches.
The team studied the common bacterium Moraxella catarrhalis, which causes middle ear infections in young children, and is a major cause of morbidity in those with heart disease. For many years, scientists approached this problem from the molecular medicine approach - through isolating and studying proteins from the Moraxella cell surface that initiate infection.
From these detailed studies the team have been able to develop an overview of one of the key proteins, called UspA1. However, as with the vast majority of molecular medicine approaches, this model has been based on studies of the UspA1 protein in isolation, rather than in its natural setting on the bacterium surface. A common worry for many biomedical scientists is how such understanding translates into the reality of these tiny molecules when they are part of a much larger cell. Understanding the increased complexity of individual molecules within the cellular mêlée is crucial to understanding why many promising drugs fail to live up to expectations.
To begin bridging this gap in our understanding, Professors Brady and Virji teamed up with Dr Massimo Antognozzi from the University's School of Physics, whose group have been developing a novel form of atomic force microscope, termed the lateral molecular force microscope (LMFM).
Together, they have evolved the design of the LMFM microscope to optimise its ability to measure biological phenomena such as changes in UspA1 directly at the Moraxella cell surface. The LMFM differs from more conventional atomic force microscopes in tapping samples (in this case, individual cells) against an extremely fine lever, equivalent to the stylus of a record player, rather than moving the lever as is usually the case. Fabrication of extremely thin but stiff cantilevers together with exceptionally fine motor movements and a specialised visualisation system have all been combined in the device to tremendous effect. The sensitivity achieved has been further enhanced by its location within the extremely low vibration environment provided within the University's innovative Nanoscience and Quantum Information building. The result has been a machine that can measure exquisitely fine molecular changes and forces in individual molecules directly on a living cell surface.
In the Moraxella study, this development has enabled the research team to correlate intricate, atomic level detail of UspA1 obtained by X-ray crystallography of isolated fragments of the protein with delicate and previously unobservable physical changes of the bacterial cell as it binds to and infects its target human cells.
Professor Brady said: "The findings have triggered the development of a novel technology that promises to open up a new approach for studying molecular medicine. This breakthrough will undoubtedly prove equally useful for the study of many other biological processes directly within their cellular environment, something that has long been needed in molecular medicine."
This combined study has enabled the researchers to observe the very first responses as a bacterium binds to a human cell, hence opening the door to understanding the complexity of infection processes.
The UspA1 LMFM studies have been funded by the Wellcome Trust and the Biotechnology and Biological Sciences Research Council (BBSRC) and were published recently in the journal Proceedings of the National Academy of Sciences (PNAS).
Source: Bristol University