
Top stories






More news














Epidemiologists, ecologists and veterinarians say zoonoses — germs that can spread from animals to humans — are emerging in part because of unsustainable agricultural practices aimed at meeting the demand for animal protein from growing populations.
In the global South, meat production increased 260% in the past 50 years, with milk production up 90% and egg output up 340%.
The best chance we have of effectively dealing with another coronavirus or similar infectious pathogen is at the beginning — when spillover occurs — and by ensuring global coordination. - Sophie Von Dobschuetz, global surveillance coordinator, Food and Agriculture Organization
"Most emerging diseases over the last century have come from intensive agriculture, not wet markets," Delia Grace Randolph, a professor of food safety systems at the University of Greenwich and a contributing scientist at the Kenya-based International Livestock Research Institute (ILRI), tells SciDev.Net. Wet markets sell fresh foods, as opposed to dry, packaged goods, and some sell live fish and animals.
Randolph is the lead author of a new report published jointly by ILRI and the UN Environment Programme (UNEP), which notes that about 60% of human infections are estimated to have an animal origin.
Of the new and emerging human infectious diseases, about 75% "jump species" from animals to people. Most zoonoses happen indirectly, through insects such as mosquitoes or, more commonly, through food systems.
Randolph and co-authors say the "vast majority" of animals involved in zoonotic outbreaks are domestic livestock or pets — domesticated animal species share an average of 19 zoonotic viruses with people, while the average for wild animals is just 0.23 viruses, they say.
As the global human population continues to surge, the number of domesticated animals that provide protein-hungry societies with food and the number of animals, such as rats, that thrive in such environments have also increased, the report says.
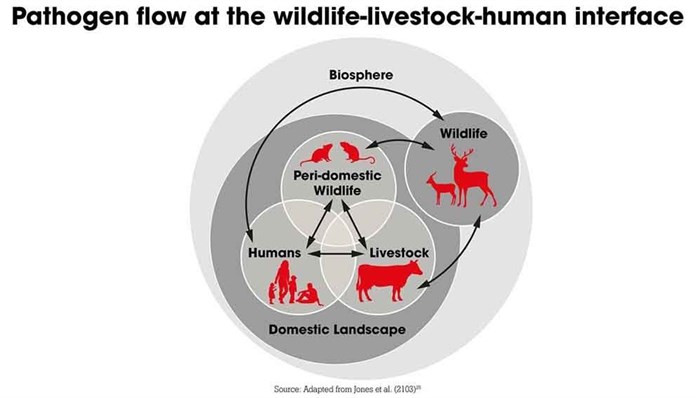
Wild habitats have become settled by human populations, bringing people and livestock into closer proximity with wildlife, notes UNEP executive director Inger Andersen. "Once established in humans, these diseases quickly spread across our interconnected world, as we have seen with Covid-19," Andersen says.
Low- and middle-income countries’ food supply chains are lengthening and diversifying. There is increased demand for animal protein and agricultural intensification is poorly regulated, all of which creates additional opportunities for disease transmission, notes the report.
The burden of neglected zoonotic diseases falls heaviest on the world’s poor, vulnerable and marginalised people, the report says. Animal-human disease control programmes need to find ways to reduce the barriers that disadvantaged groups face in managing diseases in their animals and improve access to disease control services.
Food systems must be transformed by improving policy, regulation and the monitoring of traditional food markets, because while some recent zoonotic pandemics originated from wildlife, many also came from livestock. "Our food system is good at delivering large amounts of calories to ever-increasing populations," says Randolph. "It is bad at reducing health externalities, such as obesity and disease.
"If we continue with our current food systems we will inevitably have more emerging disease."
The global food system "needs to be improved," Randolph says, "but it is not broken."
Agriculture and meat production are significant contributors of greenhouse gases, both directly and through land-use change — Andersen notes that the drivers of pandemics are often the same drivers of climate change and biodiversity loss.
Accelerating climate change has made conditions more conducive to the spread of infectious and mosquito-borne diseases that plague poor communities, such as malaria and dengue fever, and trypanosomiasis — sleeping sickness — which is spread by tsetse flies.
"Climate change results in changing environmental conditions, which impacts on the ecosystem characteristics and as a result, it changes the distribution of animal species, and therefore also of any microorganisms which they carry," says Dirk Pfeiffer, a professor of veterinary epidemiology at City University of Hong Kong and the Royal Veterinary College in London.
"The worry associated with these changes is that we struggle to make meaningful predictions in terms of what will happen."
A pivotal 2017 study published in Nature Communications mapped global hotspots for zoonotic spillover, focusing on wildlife as the origin. It argues that the risk of emerging zoonotic diseases is greatest in forested tropical regions experiencing land-use changes related to agriculture, logging, mining and infrastructure development. The study also found that the risk of zoonotic disease outbreak is high in areas rich in mammal species diversity and notes that "that the relationship between biodiversity and disease risk is complex, context-specific and idiosyncratic".
But, understanding of zoonotic spillover is incomplete and veterinary epidemiologists hesitate to say where the next zoonotic pandemic will originate. Experts are still working to determine how Covid-19 and other coronaviruses, such as the 2003 Severe Acute Respiratory Syndrome (SARS) virus, emerged. The possibilities of genetic mutations, or the viral recombination of genetically unrelated virus strains, are also alarming scientists.
"Gaps in virus understanding, early reporting, and immediate response all present huge bottlenecks," says Sophie Von Dobschuetz, global surveillance coordinator at the Food and Agriculture Organization (FAO).
"The best chance we have of effectively dealing with another coronavirus or similar infectious pathogen is at the beginning — when spillover occurs — and by ensuring global coordination."
Global efforts to reduce the impacts of emerging diseases are largely focussed on post-emergence outbreak control, quarantine, treatment and vaccine development. But, in the past decade a range of projects have been launched to identify existing viruses in animals and better understand the interface between wildlife, livestock and human interactions.
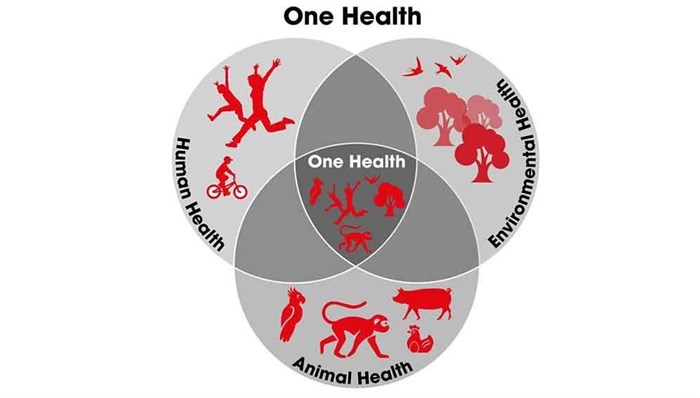
The University of California, Davis PREDICT project, part of USAID’s Emerging Pandemic Threats programme, aims to strengthen and support global surveillance and diagnostic capabilities, primarily in Africa and Asia. PREDICT says it has trained almost 7000 people in more than 30 countries in One Health — a transdisciplinary, collaborative approach to understanding zoonotic diseases risks. Still, more investment in early warning and preparedness is a priority, says Von Dobschuetz, to include the animal and wildlife health sectors in the One Health approach. The FAO and the WHO, along with the World Organisation for Animal Health (OIE) are adopting the approach.
Improved and sustained collaboration between medical, veterinary and wildlife authorities is key and partnerships need to be institutionalised to ensure collaboration beyond crisis periods, advocates say.
Meanwhile, Randolph and colleagues report that interventions to curb zoonotic diseases in animals, which were promising at the project level, are often not adopted by development programmes or the public sector. They emphasise the need for evidence-informed policy to better understand the complex risks between humans and livestock, as well as the possibilities for action to reduce diseases.
This article was published on SciDev.Net sub-Saharan Africa.