African countries need cheaper Covid-19 tests: here's how to get them

Testing detects infected individuals, especially the 45% of people who do not show symptoms, but can spread the disease. Testing also helps us to know who has recovered from the disease to fully grasp its impact. It is a key part of a multi-pronged approach to control the Covid-19 pandemic.
An ideal test for the diagnosis of Covid-19 would be cheap, accurate and easy to use. But most real tests are only one – not all three. The art is in choosing a test whose strengths and limitations are aligned with what a population needs and can afford.
For developing countries, the ideal test must be sensitive and specific enough to be clinically meaningful, and cheap enough to be implemented at scale by every country. Africa and Asia are far behind in testing. Most countries in Africa and Asia have tested less than 1% of their populations. In contrast the US, Russia and every country in Europe had tested 10%-20% of their populations by June 2020.
Covid-19 has taken root and is spreading fast in Africa. Its testing needs to keep pace with the pandemic. The nucleic acid tests that African countries have so far been relying on are too complex and expensive to achieve the required level of testing. Lower cost antigen tests are the answer. Governments and stakeholders should pursue the use of quality-verified antigen-based tests to fight Covid-19. The sooner such affordable and scalable testing strategy is adapted the better.
The options
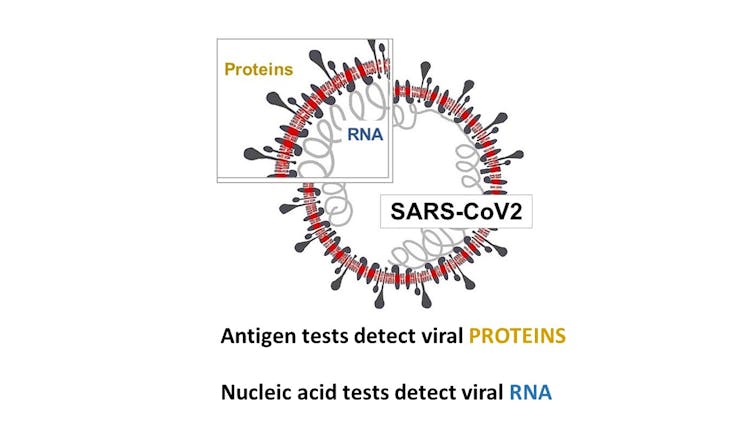
There are two types of tests that can detect early Covid-19: nucleic acid and viral antigen-based tests. Nucleic acid tests detect the viral nucleic acids. Antigen-based tests detect the viral spike of coronal proteins from which the virus derives its name. Far more nucleic acid tests than antigen-based tests have been developed and approved.
Read more:
Pasha 60: The challenges facing African countries testing for Covid-19
The global scale-up in testing for Covid-19 has almost solely relied on complex and expensive nucleic acid tests. In our opinion, this is a mistake.
Generally speaking, the best nucleic acid tests are 10%-20% more sensitive than the best antigen tests, but are more complex, and cost five to 10 times as much. For example the average charge in the US for the hepatitis B surface antigen is $56 while that for hepatitis B viral load (nucleic acid) is $409. This higher cost and complexity makes it challenging to scale up nucleic acid testing, even in a wealthy country.
Read more:
COVID-19 testing: the answers to six simple questions
The problem is global, but the impact is not the same everywhere. For wealthy countries, a lack of antigen-based testing means a more cautious reopening. But for low and middle-income countries, which account for 84% of the world’s population, a lack of antigen-based tests in the marketplace means little to no testing, unrestricted disease spread, and death.
Why antigen-based tests are scarce
By June 2020, only 20 of the more than 700 tests listed by the Foundation for Innovative New Diagnostics, a global non-profit organisation, were antigen-based tests. And only one antigen-based test was authorised for use in the US, compared to over 100 nucleic acid-based tests.
If antigen-based tests could help, why are they not being developed by diagnostics companies?
The first and most often cited reason is their relatively lower sensitivity. The single antigen-based test that has been approved by the Food and Drug Administration in the US has a sensitivity of 80% relative to nucleic acid tests. In Spain, attempts to adopt antigen tests for rapid screening were abandoned because the tests only detected 30% of positive samples. In keeping with this the World Health Organisation (WHO) has released guidance urging caution in the adoption of these tests.
Read more:
COVID-19: to test or not to test
A second and less often cited reason for the lack of antigen-based tests is that they represent a less attractive long term investment for companies that diagnose diseases from samples (in vitro diagnostics). Nucleic acid-based tests are often designed for specific machines which can be used for other tests once the disease is under control. Contrary to this, Covid-19 antigen tests are likely to lose relevance in the post-pandemic period once there is no longer a need for diagnosing early disease.
The WHO’s call for caution is appropriate but should not be construed as a reason to end the development of all antigen-based tests. Rather, any concerns about the test performance should be addressed in one of two ways.
First, the type of test kit should be rigorously verified to make sure it performs as advertised. This process of verification is well known and already used by laboratories around the world, and could be done by a central body like the Foundation for Innovative New Diagnostics or Africa Centres for Disease Control and Prevention.
Second, antigen-based tests can be used in combination with nucleic acid tests. A positive antigen-based test is reliable. But if there seems to be a high risk that a person is positive even though the antigen test shows a negative result, then the additional, more sensitive, more expensive nucleic acid test can be done. This approach has just been adopted by the Indian Council of Medical Research and is appropriate for other low and middle-income countries.
One way to mitigate the financial risk of test development for diagnostic companies might be for health ministries, NGOs and other stakeholders to support the development costs of antigen-based tests. There are previous examples of this, such as the GeneXpert system, which diagnoses TB.
Decision-making around testing is complicated, and neither nucleic acid nor antigen-based testing is an ideal option in every country. What we are arguing for is choice. Wealthy countries have made the choice to pay more for testing to avoid a higher rate of false negatives. For most of the world, the expense means there is no choice, and the consequence is little or no testing. That in turn means more deaths and other impacts of this pandemic.
The global search for Covid-19 diagnostics must devote a larger share of attention and resources to developing and vetting antigen-based tests, because they are the best option for rapid scale-up for the majority of the world’s population.
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()
Source: The Conversation Africa

The Conversation Africa is an independent source of news and views from the academic and research community. Its aim is to promote better understanding of current affairs and complex issues, and allow for a better quality of public discourse and conversation.
Go to: https://theconversation.com/africa