Testing the power of regenerative medicine to treat eye diseases, surgeons at UCLA's Jules Stein Eye Institute on Tuesday successfully transplanted highly specialised cells derived from human embryonic stem cells into the eyes of two patients.
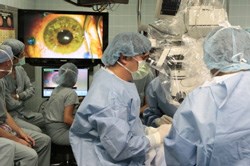
Dr. Steven Schwartz peers into a microscope during surgery to transplant highly specialised cells derived from human embryonic stem cells into the eyes of the first patients enrolled in two clinical trials that are testing the promise of stem cell therapy. (Image: Reed Hutchinson)
Both patients, one with Stargardt's macular dystrophy and the other with dry age-related macular degeneration, underwent outpatient transplantation surgeries this week and are recovering uneventfully, according to the surgeon, Dr. Steven Schwartz, retinal division chief at Jules Stein (JSEI) and the Ahmanson Professor of Ophthalmology at the David Geffen School of Medicine. Schwartz is the principal investigator on two clinical trials, one for each eye disease; each trial will include 12 patients, who are legally blind, and will determine the safety of stem cell therapy as well as the patients' ability to tolerate the treatment.
"Early indications are that the patients tolerated the surgical procedures well," said Dr. Schwartz in a statement. Advanced Cell Technology, Inc. (ACT) of Marlborough, Mass., a leader in the field of regenerative medicine, has been working for the last decade on developing a stem cell therapy to treat eye diseases.
An important milestone
Human embryonic stem cells can differentiate into any cell type. The stem cell-derived retinal pigment epithelial (RPE) cells that were transplanted during surgery were differentiated in ACT labs. Each patient received a relatively low dose of the transplanted RPE cells (50 000) into the subretinal space of the treated eye.
The dosing of the first patients in these trials, which are being closely watched by scientists and stem cell therapy advocates around the world, was hailed by company officials as an important milestone in the therapeutic use of stem cells and may pave the way for a new therapeutic approach to treating eye diseases.
Hopes that the transplanted RPE cells will implant and begin functioning
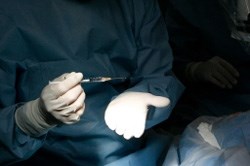
The surgeon holds a syringe with cells that were prepared in the lab of the biotechnology company that developed the therapy, Advanced Cell Technology. (Image: Reed Hutchinson)
Currently, both eye diseases are untreatable. The dry form of macular degeneration, the most common form of the disease, is the leading cause of blindness in the developed world, especially among people over the age of 55. As many as 30 million people in the United States and Europe suffer from this disease. However, the number of people affected is expected to double over the next 20 years as the population ages. Stargardt's causes progressive vision loss, usually starting when patients are between 10 to 20 years of age.
In both conditions, the layer of RPE cells located beneath the retina deteriorates and atrophies. These cells support, protect and provide nutrition for light-sensitive photoreceptors in the eye. Over time, the death of the RPE cells and eventual loss of the photoreceptors can lead to blindness as central vision is gradually destroyed. Doctors are hoping the transplanted RPE cells will implant and begin functioning.
"Today - 13 years after the discovery of human embryonic stem cells - the great promise of these cells is finally being put to the test," said Dr. Robert Lanza, chief scientific officer of ACT, who attended both procedures at Jules Stein. "The initiation of these two clinical trials marks an important turning point for the field. ... It's time to start moving these exciting new stem cell therapies out of the laboratory and into the clinic."
Source: UCLA






































