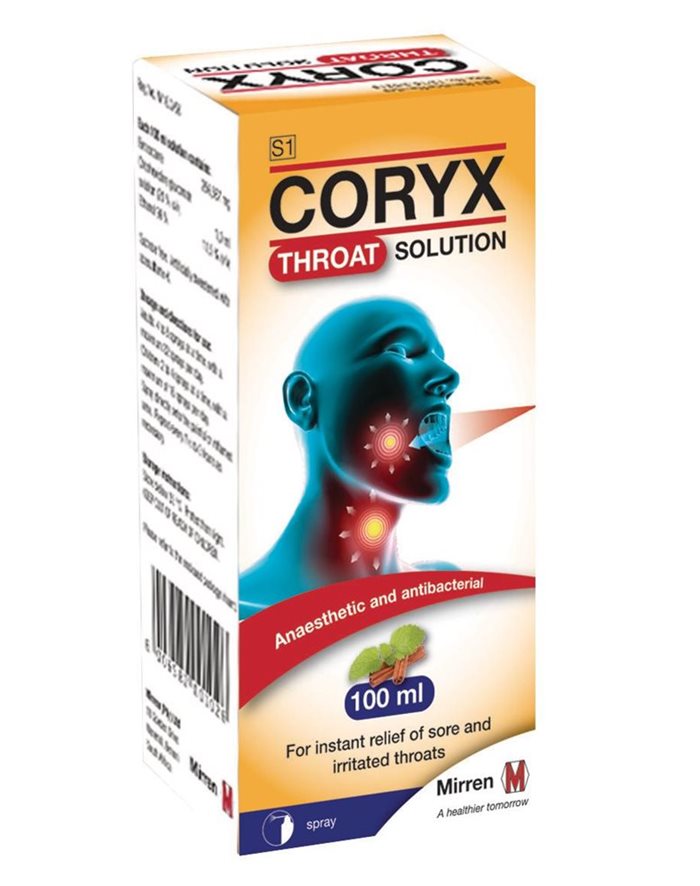
Cipla Medpro, in collaboration with the South African Health Products Regulatory Authority (SAHPRA), is recalling some batches of Coryx throat spray.
Source: Supplied.
The reason for the Class I Type A product recall is due to the possibility of the spray nozzle detaching from the spray mechanism during use, resulting in the spray nozzle being ingested or getting stuck in the throat after detaching.
The batches being recalled are: ZB000156, ZB000157, ZB000158, ZB000159, ZB000160, as well as ZB100105, ZB100107, ZB100172 and ZB100173.
People who purchased any of these batches of Coryx throat spray are requested to return it to their pharmacy, and they will be refunded the purchase price.
The chief executive officer of Cipla South Africa, Paul Miller, said: “The safety and wellbeing of our patients is our utmost priority. Therefore, after liaising with SAHPRA, it was deemed necessary to recall specific batches of this product from the market.
"We are working closely with spray-mechanism manufacturers to ensure there is no margin for error in future.”



































