South Africa didn't take advantage of its role in Covid-19 vaccine trials: why it should have
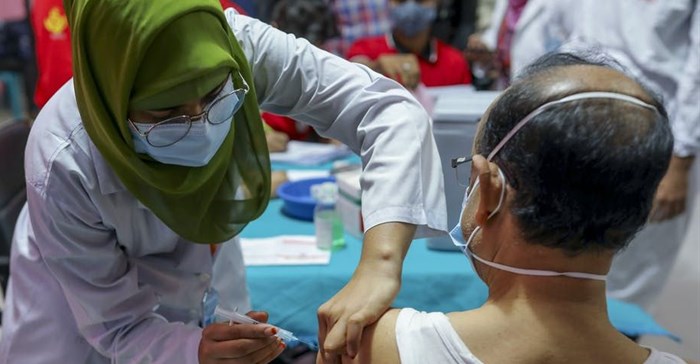
More than 100 million doses of Covid-19 vaccines have been administered globally to date. Only about 200,000 (0.2%) have been in Africa.
South Africa is one of the first countries in sub-Saharan Africa to procure vaccines. The country has some of the highest reported cases of Covid-19 in the region. But, despite hosting a clinical trial of the AstraZeneca vaccine, South Africa was unable to secure a fair pricing agreement.
The country procured its first delivery of the AstraZeneca vaccine from the largest vaccine manufacturer in the world, the Serum Institute of India. A million doses of the vaccine arrived at a cost levied by AstraZeneca of $5.25 per dose. This is more than double the $2.16 per dose paid by European Union countries to AstraZeneca.
It was disappointing for research participants and other citizens alike. By not leveraging South Africa’s participation in clinical trials, authorities violated the well established fundamental principles of post-trial access and benefit sharing in research.
Post-trial access and benefit sharing can apply to participants in a specific trial. But they can also extend to the community in which the research was conducted.
There’s no international policy guideline that specifically addresses the scope and nature of benefits on a national scale. Some may argue that the scientists who conduct clinical trials shouldn’t negotiate for benefits. But we argue that in a pandemic situation, sponsors, researchers and research ethics committees have an obligation to ensure the highest scientific standards and to negotiate post-trial benefits in advance.
Access and benefit sharing
Post-trial access and benefit sharing are firmly entrenched research ethics principles. They are part of collaborative partnership, which guides the ethical conduct of research in developing countries. Collaborative partnership requires a fair distribution of tangible and intangible rewards of research among the partners.
Resentment, mistrust, and a sense of exploitation are inevitable if research participants do not benefit.
The ethics community and international documents agree in principle that individuals and communities that participate in research ought to benefit. This is especially true when the product has commercial value and the study was conducted in resource-limited countries with many healthcare challenges.
HIV research is an example. New guidance from the Joint United Nations Programme on HIV/Aids recommends that, before a trial starts:
health and research communities… should initiate a process of discussion and negotiation about how products will be made available to the country in which the products are tested if the HIV preventive intervention is efficacious.Sponsors of vaccine trials should at least give the placebo group whatever is proven to work. Ideally, a plan of wider access to the population at risk should be negotiated. One can argue that this obligation is greatest when it’s the only intervention that exists and lives depend on it. Subsidised access for host communities could reduce inequalities between countries and ensure fairness in the research process. Access to HIV treatment in Africa is an example of where this has been successfully negotiated.
Ordinarily, post-trial access can be delayed by local product registration. It can take around five years to have a medical product or device registered. In contrast, emergency use authorisation of Covid-19 vaccines has made products available much sooner – weeks to months after trial completion. This increases the possibility of successfully negotiating post-trial access for participants, communities and, in a pandemic, even for countries.
Collective responsibility
Given the complexity of vaccine procurement, this responsibility must be collective. It falls on global health institutions, national governments, researchers, research ethics committees and even research participants.
Some researchers, while responsible for ensuring the highest level of scientific integrity, seem to have drawn a false dichotomy between their scientific obligations and their ethical responsibilities to their study participants, communities and country. They suggest negotiating for post-trial access to vaccines could be seen as a conflict of interest or an unfair inducement. But we don’t agree.
In our view, negotiating for fair treatment before a study begins is ethically necessary.
The International Conference for the Harmonisation of Good Clinical Practice guidelines regulate the conduct of clinical trials globally. These guidelines require researchers to be responsible for maintaining the highest levels of scientific research and ethical values. One of these values is justice – which means that those who bear the burden of research must benefit.
These guidelines ensure that the science is robust while also paying necessary attention to the ethics. Therefore negotiating for access cannot be viewed as a perverse incentive.
Research ethics committees have a duty during pandemics to insist on a benefit sharing plan for urgent and important research, especially for clinical trials conducted in the public interest.
No one is safe from Covid-19 until everyone is safe. The world is in this together and low- and middle-income countries should not be left behind. It is hoped that as research continues with the Johnson and Johnson vaccine in South Africa, fair pricing agreements have already been negotiated.
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()
Source: The Conversation Africa

The Conversation Africa is an independent source of news and views from the academic and research community. Its aim is to promote better understanding of current affairs and complex issues, and allow for a better quality of public discourse and conversation.
Go to: https://theconversation.com/africa





























