
Top stories



LifestyleWhen to stop Googling and call the vet: Expert advice on pet allergies from dotsure.co.za
dotsure.co.za 2 days

AutomotiveHilux Custom Builds offers purpose-built solutions for your business
Toyota South Africa Motors 1 day
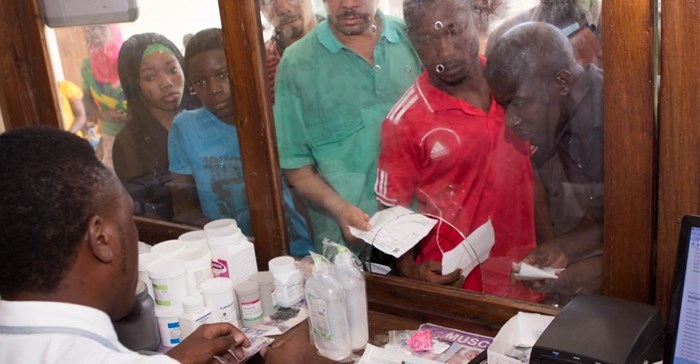
“This is a major African milestone and we are very proud of Tanzania’s achievement, which we hope will inspire other countries in the region,” says Dr Matshidiso Moeti, WHO regional director for Africa. “Access to medicines alone, without quality assurance, is not enough. With this milestone Tanzania makes a big step towards improving the quality of its healthcare services.”
Medicines are used to prevent illnesses and treat diseases, helping many people to lead full and productive lives. However, if produced, stored or transported improperly, if falsified, or used incorrectly or abused, medicines can be hazardous and can lead to hospitalisation and even death. For these reasons, it is important to have effective regulatory systems that also serve to promote timely access to quality medicines.
Fewer than 30% of the world’s medicines regulatory authorities are considered to have the capacity to perform the functions required to ensure medicines, vaccines and other health products actually work and do not harm patients. For that reason, WHO and African governments have intensified efforts to bolster the capacity of regulating medicines in the region.
Over the past years, WHO has been supporting African countries, including Tanzania to strengthen their regulatory entities.
“The core of WHO’s work is to empower countries through support and knowledge transfer so that they can expand access to health services for their populations,” says Mariângela Simão, WHO assistant director general for access to medicines, vaccines and pharmaceuticals. “If countries want to improve health outcomes, they first need to ensure access to safe and quality medical products that actually work and benefit patients.”
WHO’s assessment of regulatory authorities is based on the Global Benchmarking Tool – an evaluation tool that checks regulatory functions against a set of more than 200 indicators – such as product authorisation, market surveillance and the detection of potential adverse-effects – to establish their level of maturity.
The benchmarking of Tanzanian regulatory authorities was carried out in phases by a WHO-led team of international experts. Earlier this year, WHO facilitated self-assessments and conducted a formal evaluation of the Tanzania Food and Drug Authority (TFDA) on the mainland and the Zanzibar Food and Drug Agency and required the regulatory authorities to make a number of adjustments. In the last assessment, Tanzania FDA met all indicators that define a maturity level 3 agency, the second highest on WHO’s scale and the target for regulatory systems globally.
Established in July 2003, the Tanzania FDA has come a long way to becoming a recognised leader in medicines regulation in Africa. The latest achievement means that medical doctors, pharmacists, chemists and technicians working for the regulatory authority possess the expertise and hands-on skills to evaluate medical products, prevent and counteract associated hazards and are capable of protecting the public from substandard and falsified medicines.